Childhood Flu Vaccination Service
Community Pharmacy England (CPE) has announced that a Childhood Flu Vaccination Advanced Service, for children aged 2-3 years, will be launched from 1st October 2025, initially as a one-season trial – see here. The service specification for the service can be downloaded here.
The patient group direction (PGD) for the service has been approved and published by NHS England. The PGD can be downloaded here.
Pharmacy owners that choose to sign up to provide the service will be able to do so via the NHSBSA’s MYS portal from 1st August 2025. Please ensure that you read the service specification and can meet all the minimum requirements before signing up to provide the service.
In order to receive vaccines ahead of the start of the service on 1st October 2025, pharmacy owners will need to register on MYS by 11:59pm on 31st August 2025. Later registration will be possible, but the provision of the centrally procured vaccine will be received after the starting date for the service.
For all the latest and most up-to-date information on the Childhood Flu Vaccination Service, refer to the CPE webpage here.
Eligible Patients
The Annual flu letter and the Update to the Annual flu letter confirm the eligible patient cohorts for the 2025/26 season.
The Childhood Flu Vaccination Community Pharmacy service covers all children aged 2 and 3 years of age on 31st August 2025 – that means children who were born on or after 1st September 2021 and on or before 31st August 2023.
All children aged less than 2 years of age or aged four years or over on 31st August 2025 are not covered by the service.
Vaccines
First Line
Most eligible children will be vaccinated with Live Attenuated Influenza Vaccine (LAIV) nasal spray suspension which is the first line vaccine. This vaccine will be supplied by NHSE from centrally procured stock through the NHSE Federated Data Platform (FDP). Pharmacy owners must register for FDP to be able to order vaccines.
Guidance on how to register to access the FDP can be found here.
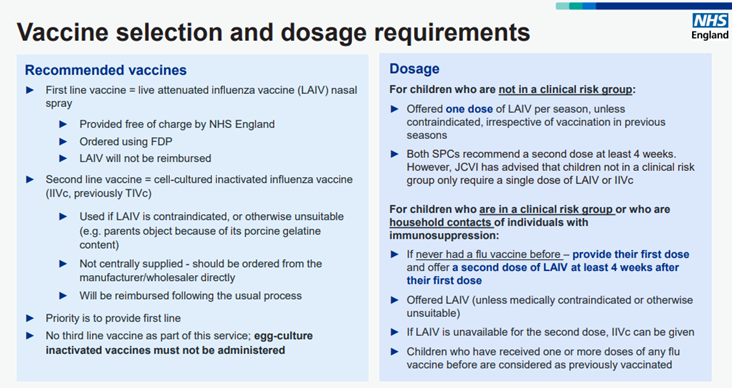
Second Line
When LAIV is contraindicated or otherwise unsuitable (for example, where parents object to LAIV on the grounds of its porcine gelatine content), Cell-Cultured Inactivated Influenza Vaccine (IIVc) should be used. This will not be centrally supplied and should be obtained by placing an order via manufacturers or wholesalers, in line with the normal approach to purchasing flu vaccine.
The reasons for use of the second line vaccine should be documented in the clinical record – evidence of this may be requested by NHS England before reimbursement is agreed. The annual flu letter does include a third line vaccine, but this cannot be administered as part of this service.
Resources
For the Pharmacy Team
- CPE Childhood Flu Webinar Slides (20th August 2025)
- CPE on-demand Childhood Flu webinar – see here.
- UKHSA Poster – Flu Vaccines for Children and Young People 2025-26
For Patients
Paper copies of this leaflet are available to order for free or download on the Health Publications website using the product code 2025FCEN.
This leaflet is available to order and download in the following languages: Albanian, Arabic, Bengali, Bulgarian, Chinese (Simplified), Chinese (Traditional), Dari, Estonian, Farsi, French, Greek, Gujarati, Hindi, Italian, Latvian, Lithuanian, Nepali, Panjabi, Pashto, Polish, Portuguese, Romanian, Romany, Russian, Somali, Spanish, Tagalog, Tigrinya, Turkish, Twi, Ukrainian, Urdu, Yiddish, and Yoruba.
- This link can be shared with parents/carers of children eligible for the flu vaccine to support promotion of the service: see UKHSA – protect your child against flu
- Leaflet- Vaccines & Porcine Gelatine. Translations of this leaflets are available to download in: Arabic, Bengali, Gujarati, Panjabi and Urdu.
- Stickers – are available to order (free) using product code SCHFLSTK to give to children who have received a flu vaccine.
Training
Training
- NHS Education for Scotland has made a video for healthcare professionals on how to administer the Fluenz nasal spray including to children aged 2-5 years.
- NHSE has produced this resource pack (link below) which signposts to useful information, training and resources:
Flu Vaccination for Children 2025/26 – Training and Resources
- CPPE has also added a new section dedicated to the NHS Childhood Flu Service – see Vaccination Services : CPPE
Last Updated: 11th September 2025



